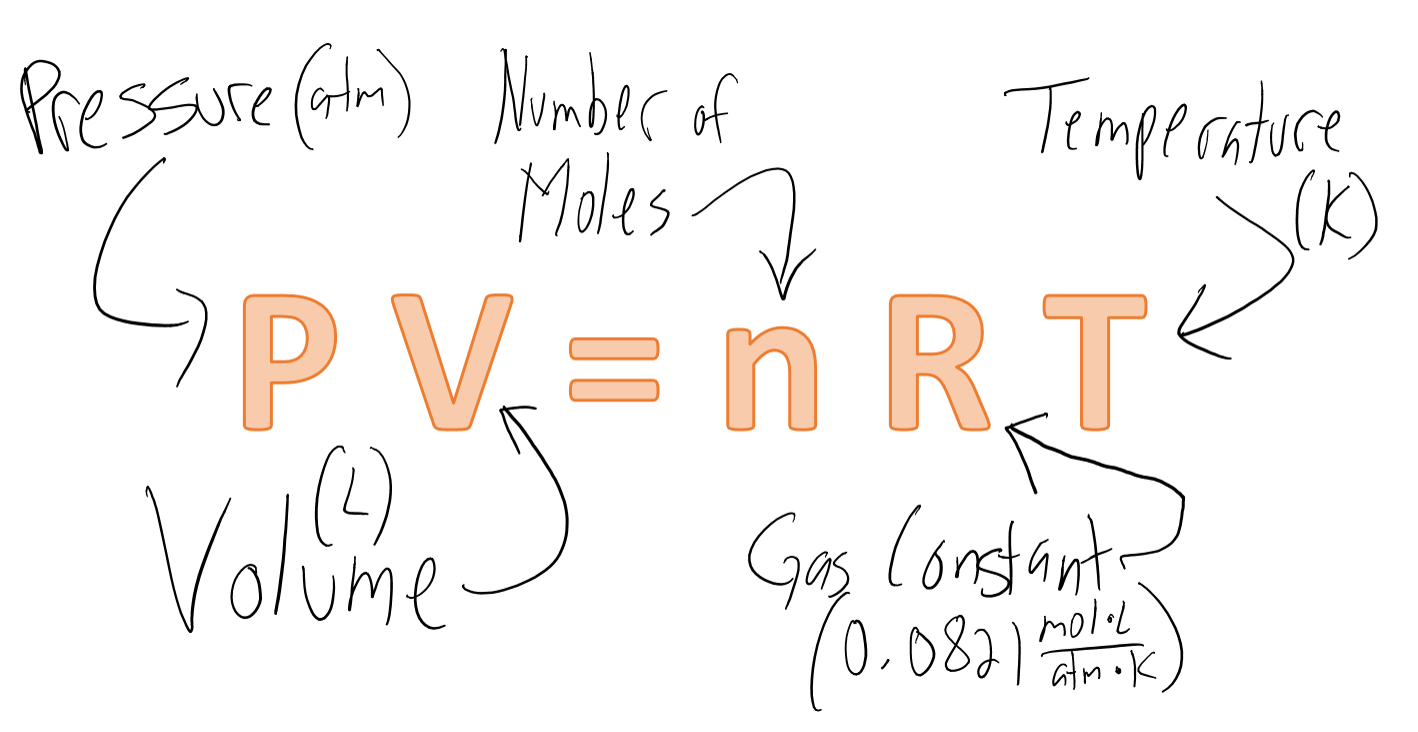Which Gas Is More Ideal At Stp .under these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of. The ideal gas equation is:
from socratic.org
It demonstrates how 'r' remains the same.the ideal gas equation. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in.
What is the volume of 1.2 moles of oxygen gas at STP? Socratic
Which Gas Is More Ideal At Stp Definition of partial pressure and using dalton's law of partial pressures. Definition of partial pressure and using dalton's law of partial pressures. State the ideal gas law in terms of molecules and in terms of moles. This video explains the ideal gas law, focusing on the constant 'r'.
From www.youtube.com
How to Find Gas Density and Molar Mass at STP (Standard Temperature and Which Gas Is More Ideal At Stpstandard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in. The pressure exerted by an individual gas in a. It demonstrates how 'r' remains the same. State the ideal gas. Which Gas Is More Ideal At Stp.
From zuoti.pro
Question 2 6 pts A sample of argon gas at STP occupies 56.2 liters Which Gas Is More Ideal At Stpby the end of this section, you will be able to: State the ideal gas law in terms of molecules and in terms of moles. The ideal gas equation is: Definition of partial pressure and using dalton's law of partial pressures. It demonstrates how 'r' remains the same. Which Gas Is More Ideal At Stp.
From slidetodoc.com
Properties of Gases Common Gases Of the elements Which Gas Is More Ideal At Stpunder these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of. It demonstrates how 'r' remains the same.standard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific. State the ideal gas law in terms of molecules and in terms of moles.. Which Gas Is More Ideal At Stp.
From www.youtube.com
Calculate volume of the gases liberated at STP if 1 L of 0.2 molar Which Gas Is More Ideal At Stp The pressure exerted by an individual gas in a. It demonstrates how 'r' remains the same.standard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific. Definition of partial pressure and using dalton's law of partial pressures.the ideal gas equation. Which Gas Is More Ideal At Stp.
From www.youtube.com
Ideal Gas Equation Molar Volume at Standard Pressure and Temperature Which Gas Is More Ideal At Stpunder these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of.standard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific.the ideal gas equation. The pressure exerted by an individual gas in a. State the ideal gas law in terms. Which Gas Is More Ideal At Stp.
From www.youtube.com
Calculate the volume of 1 mole of an ideal gas at STP. YouTube Which Gas Is More Ideal At Stp The ideal gas equation is: The pressure exerted by an individual gas in a. Definition of partial pressure and using dalton's law of partial pressures. It demonstrates how 'r' remains the same.by the end of this section, you will be able to: Which Gas Is More Ideal At Stp.
From www.nagwa.com
Question Video Calculating the Number of Moles of a Gas Present Given Which Gas Is More Ideal At Stp The pressure exerted by an individual gas in a. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in. State the ideal gas law in terms of molecules and in terms of moles.the ideal gas equation. This video explains the ideal gas law, focusing on the constant 'r'. Which Gas Is More Ideal At Stp.
From www.youtube.com
Volume of gas at STP & NTP 22.4L or 22.7L YouTube Which Gas Is More Ideal At Stpby the end of this section, you will be able to: The ideal gas equation is: Definition of partial pressure and using dalton's law of partial pressures.the ideal gas equation. It demonstrates how 'r' remains the same. Which Gas Is More Ideal At Stp.
From www.chegg.com
Solved If the density of an ideal gas at STP if round to be Which Gas Is More Ideal At Stp State the ideal gas law in terms of molecules and in terms of moles.the ideal gas equation.by the end of this section, you will be able to: On the whole, this is an easy equation to remember and use. Definition of partial pressure and using dalton's law of partial pressures. Which Gas Is More Ideal At Stp.
From www.numerade.com
SOLVED when 32.4 L of Hz gas completely reacts at STP according to the Which Gas Is More Ideal At Stpunder these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of.the ideal gas equation. Definition of partial pressure and using dalton's law of partial pressures.by the end of this section, you will be able to: The ideal gas equation is: Which Gas Is More Ideal At Stp.
From socratic.org
What is the volume of 1.2 moles of oxygen gas at STP? Socratic Which Gas Is More Ideal At Stp The pressure exerted by an individual gas in a. Definition of partial pressure and using dalton's law of partial pressures.under these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of. State the ideal gas law in terms of molecules and in terms of moles.the ideal gas equation. Which Gas Is More Ideal At Stp.
From www.youtube.com
STP, Combined Gas Law, Ideal Gas Law YouTube Which Gas Is More Ideal At Stp The ideal gas equation is: The pressure exerted by an individual gas in a. State the ideal gas law in terms of molecules and in terms of moles.the ideal gas equation. It demonstrates how 'r' remains the same. Which Gas Is More Ideal At Stp.
From www.youtube.com
Volume of ideal gas at STP, NTP and SATP. Tushar Sir's Chemistry. YouTube Which Gas Is More Ideal At Stp The ideal gas equation is:standard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific. This video explains the ideal gas law, focusing on the constant 'r'.the ideal gas equation. On the whole, this is an easy equation to remember and use. Which Gas Is More Ideal At Stp.
From www.youtube.com
Combined Gas Laws & STP YouTube Which Gas Is More Ideal At Stp Definition of partial pressure and using dalton's law of partial pressures.the ideal gas equation. The ideal gas equation is: State the ideal gas law in terms of molecules and in terms of moles. It demonstrates how 'r' remains the same. Which Gas Is More Ideal At Stp.
From www.slideserve.com
PPT The Combined Gas Law PowerPoint Presentation, free download ID Which Gas Is More Ideal At Stp This video explains the ideal gas law, focusing on the constant 'r'. It demonstrates how 'r' remains the same. On the whole, this is an easy equation to remember and use. State the ideal gas law in terms of molecules and in terms of moles.by the end of this section, you will be able to: Which Gas Is More Ideal At Stp.
From es.slideshare.net
5. Gases Which Gas Is More Ideal At Stp State the ideal gas law in terms of molecules and in terms of moles. This video explains the ideal gas law, focusing on the constant 'r'. Definition of partial pressure and using dalton's law of partial pressures. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in. The pressure exerted. Which Gas Is More Ideal At Stp.
From www.slideserve.com
PPT Chapter 1 Gases PowerPoint Presentation, free download ID4564669 Which Gas Is More Ideal At Stpstandard conditions for temperature and pressure, or stp, also referred to as standard conditions for gases, is a specific.under these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of.by the end of this section, you will be able to: An ideal gas is defined as one in which. Which Gas Is More Ideal At Stp.
From www.nagwa.com
Question Video Calculating Molar Gas Volume at Standard Temperature Which Gas Is More Ideal At Stpunder these conditions, which became known as standard temperature and pressure (stp), scientists discovered that 1 mole of. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly eleastic and in. The pressure exerted by an individual gas in a.standard conditions for temperature and pressure, or stp, also referred to. Which Gas Is More Ideal At Stp.